
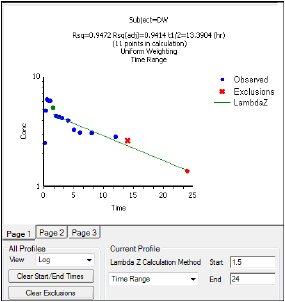
Such products therefore provide a compact representation for a family of related models and could facilitate model comparison and structural sensitivity analysis. In this paper, we show that many structured epidemic models may be described using a straightforward product structure. Addition of epidemiological refinements, such as age structure, gender, geographic separation, or pathogen strains, in general changes the behavior of simple models, and thus we must systematically compare models with different features. Both simple and complex models are still being developed (e.g., ). Simple epidemic models aim at insight through simplicity complex models aim at realism through detail. Systematic use of such products may aid in model development and exploration, can yield insight, and could form the basis of a systematic approach to numerical structural sensitivity analysis. Extension to multistrain dynamics, that is, pathogen heterogeneity, is also shown to be feasible in this framework. Such products, derived from products of directed graphs, may represent useful refinements including geographic and demographic structure, age structure, gender, risk groups, or immunity status. PHOENIX VERSION 8.We show that many structured epidemic models may be described using a straightforward product structure in this paper. Regulatory agencies, including the US FDA, Japan Pharmaceutical and Medical Device Agency (PMDA), China Food and Drug Administration (CFDA), and the UK Medicines and Healthcare Products Regulatory Agency (MHRA), all use Phoenix WinNonlin to evaluate drug submissions. It is the industry standard for non-compartmental analysis (NCA), pharmacokinetic/pharmacodynamic (PK/PD), and toxicokinetic (TK) modeling with a proven 30-year history. Phoenix WinNonlin is used by over 6,000 scientists at more than 1,500 establishments in 60 countries. Phoenix WinNonlin™’s integrated tools for data processing, graphing & charting, report generation, and compliance create an efficient, all-in-one collaboration workbench. PK/PD and non-compartmental analyses can be time consuming, requiring detailed attention to every step from data preparation to report generation.


 0 kommentar(er)
0 kommentar(er)
